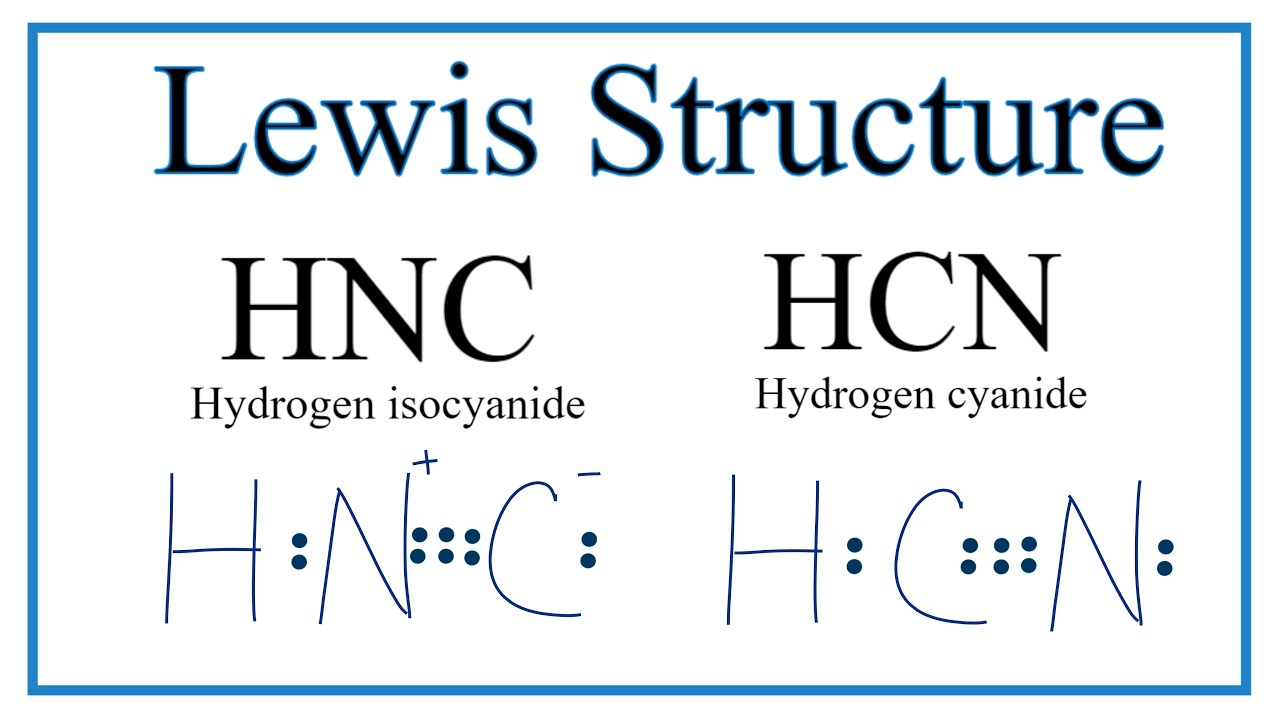
Hcn Lewis Structure Bonds Draw Easy
Periodic table In the periodic table, hydrogen lies in group 1, carbon lies in group 14, and nitrogen lies in group 15. Hence, hydrogen has one valence electron, carbon has four valence electrons, and nitrogen has five valence electrons. Since HCN has one hydrogen atom, one carbon atom, and one nitrogen atom, so…
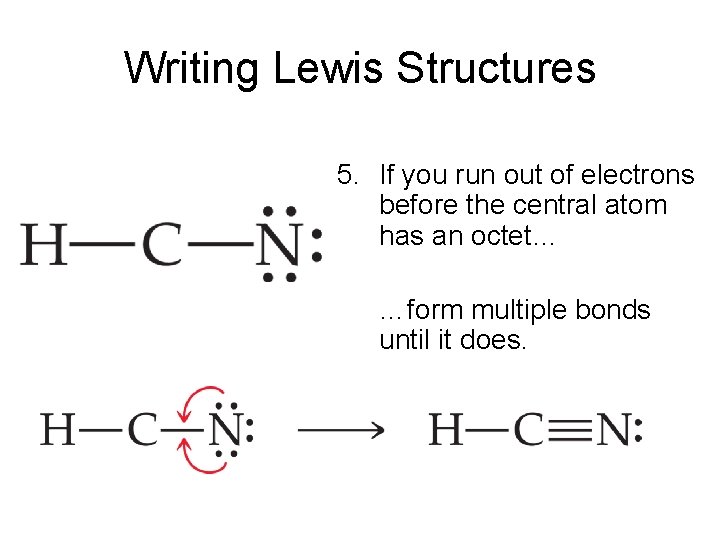
Hcn Lewis Structure Bonds Draw Easy
The molecular weight of HCN is 27.025 g/mol. The boiling point of the compound is 78.1 deg F and the melting point is 7.9 deg F. Below are the reactions or methods which lead to the creation of this compound: When methane reacts with ammonia and oxygen we get hydrogen cyanide and water.

How do you draw the Lewis structure of HCN (hydrogen cyanide)? HCN
11: Chemical Bonding
[Solved] A VB diagram of HCN is shown below (with a Lewis structure for
By Mansi Sharma HCN Lewis dot structure is of great significance in terms of understanding the number of bond pairs, lone pairs, and type of bonds involved. Though the structure seems simple many underlying complexities to are going to be discussed in this article. HCN Lewis dot structure consist of 3 elements as shown in the formula.
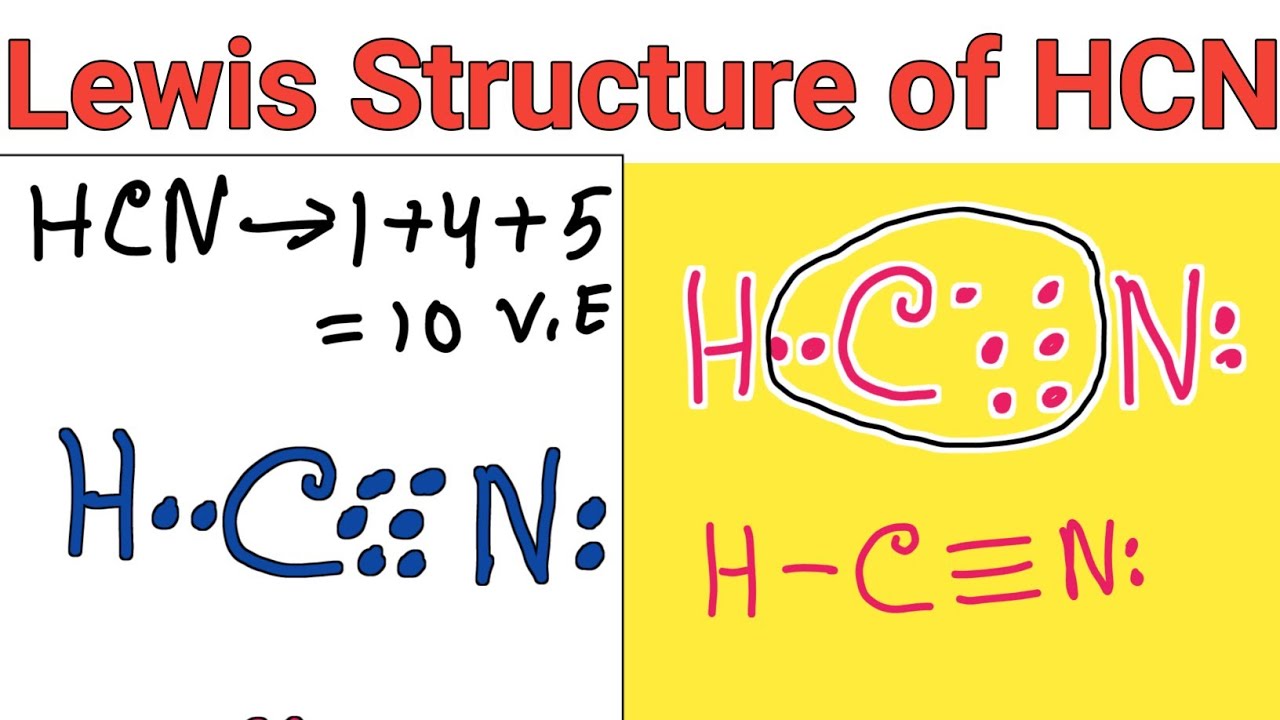
Lewis structure of HCN (Hydrogen cyanide) YouTube
Contents HCN valence electrons HCN Lewis structure HCN Molecular Geometry HCN Bond Angles HCN Shape HCN Polarity HCN valence electrons To draw the Lewis dot structure of any molecule, it is essential to know the total number of valence electrons in the structure.

HCN Lewis Structure, Molecular Geometry, Hybridization, MO Diagram, and
Calculation of valence electrons in HCN For Hydrogen: Hydrogen is a group 1 element on the periodic table. [1] Hence, the valence electrons present in hydrogen is 1 (see below image). For Carbon: Carbon is a group 14 element on the periodic table. [2] Hence, the valence electrons present in carbon is 4 (see below image). For Nitrogen:

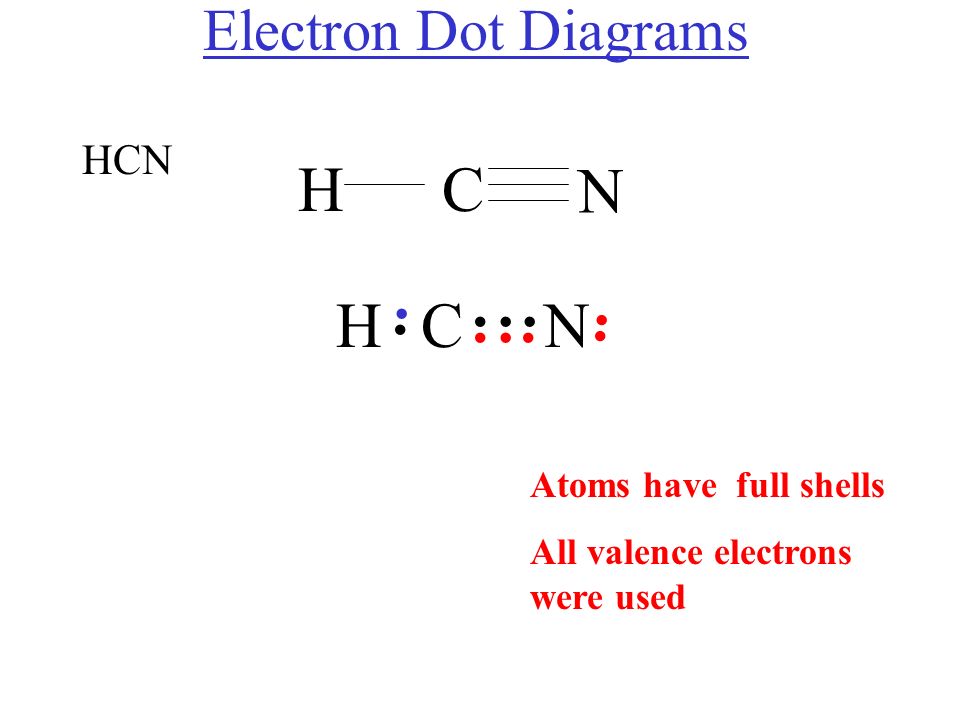
So far, we’ve used 8 of the HCN Lewis structure’s total 8 outermost
The Octet Rule The other halogen molecules (F 2, Br 2, I 2, and At 2) form bonds like those in the chlorine molecule: one single bond between atoms and three lone pairs of electrons per atom. This allows each halogen atom to have a noble gas electron configuration.
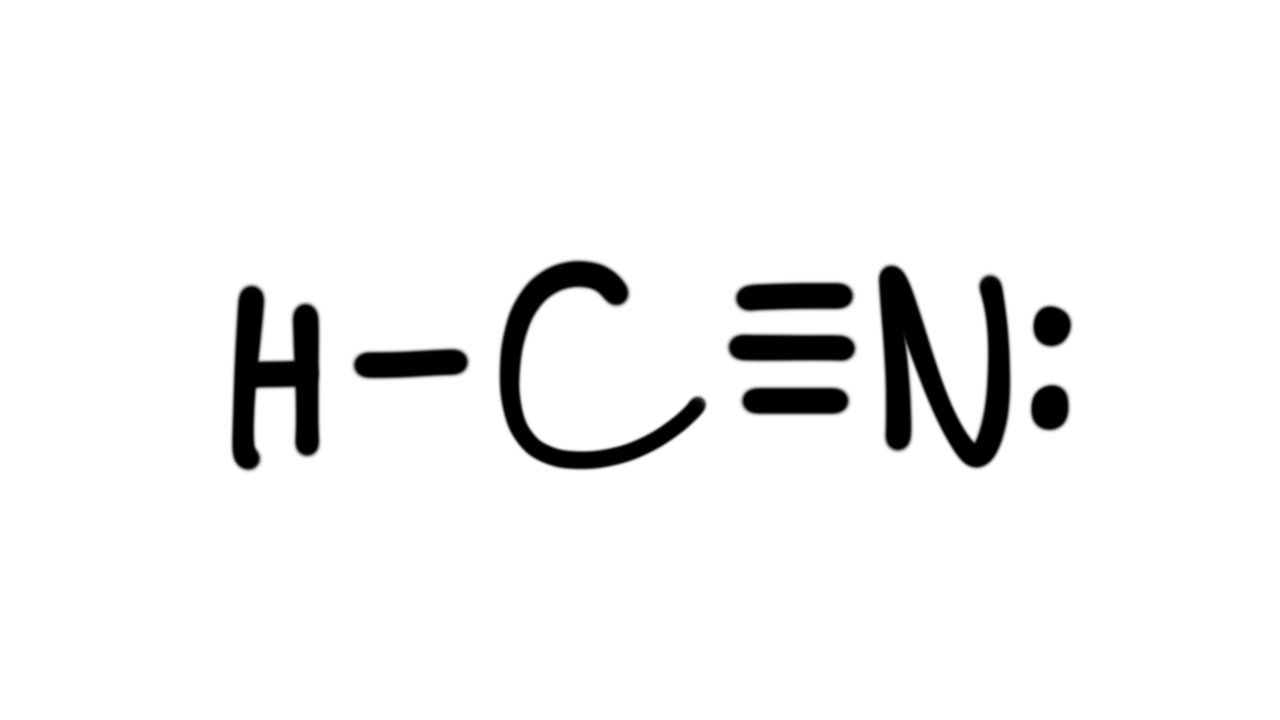
Lewis Diagram For Hcn
Lewis Structure of HCN chemistNATE 260K subscribers Subscribe Subscribed 3K Share 458K views 10 years ago Lewis Structures The Lewis Structure (Lewis Dot Diagram) for HCN. 1. Count.

Hcn Lewis Structure Bonds Draw Easy
Hydrogen cyanide (HCN)- Hydrocyanic acid is a highly poisonous solution of hydrogen cyanide in water with the formula HCN. To learn the Lewis acid Structure, Molecular mass, Physical and Chemical Properties and Uses with FAQs of Hydrogen Cyanide visit BYJU'S.

4. 11C04.1 PSV 1 Lewis structure of HCN YouTube
What is the formal charge on the HCN Lewis structure? The polarity of the molecules Lewis Structure and Molecular Geometry External Reference: Key Points To Consider When Drawing The HCN Electron Dot Structure A three-step approach for drawing the HCN Lewis structure can be used.
Lewis Diagram For Hcn
Drawing the Lewis Structure for HCN. Make sure you put the correct atom at the center of the HCN molecule. With the Lewis Structure for HCN you'll need to share more than one pair of electrons between the Carbon and the Nitrogen atoms. Be sure that you don't use more than the ten valence electrons available.

Draw The Lewis Dot Structure Of Hcn Fotodtp
0:00 / 1:15 HCN Lewis Structure: How to Draw the Lewis Structure for HCN Wayne Breslyn 729K subscribers Join Subscribe Subscribed 1.4K Share 270K views 10 years ago Lewis Structures.

Molecular Geometry, Lewis Structure, and Bond Angle of HCN
Steps for Writing Lewis Structures. Calculate the sum of the valence electrons in the molecule. 1 C atom = 1 × 4 = 4 valence e -. 1 O atom = 1 × 6 = 6 valence e -. 2 Cl atoms = 2 × 7 = 14 valence e -. sum of valence e - = 24 valence e -. Construct a skeleton structure for the molecule. C is the central atom since it makes the most.
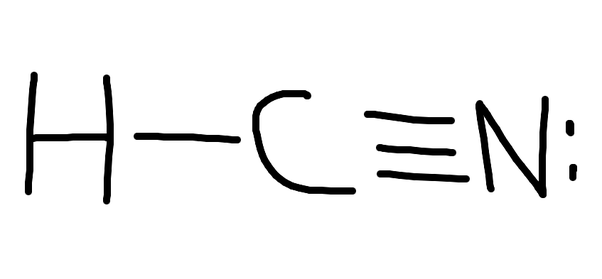
Lewis Diagram For Hcn
search Search build_circle Toolbar fact_check Homework cancel Exit Reader Mode school Campus Bookshelves menu_book Bookshelves perm_media Learning Objects login Login how_to_reg Request Instructor Account hub Instructor Commons Search Search this book Submit Search Downloads expand_more Download Page (PDF) Download Full Book (PDF)

Diagrama De Lewis Hcn Estudiar
What is the Lewis Structure of HCN? What is the Lewis Structure of HCN? The Lewis structure of HCN has carbon triple bonded to nitrogen and single bonded to hydrogen. The molecule is linear. What is this molecule and what is it used for? HCN, hydrogen cyanide, is rather poisonous.

HCN Lewis StructureHydrogen Cyanide (HCN) Lewis Dot StructureDraw
Ernest Z. Nov 12, 2017 Here's how to do it. Explanation: Step 1. Draw a skeleton structure Put the least electronegative atom C in the middle with H and Cl on either side. H-C-N Step 2. Count the valence electrons you can use H + C + N =1 + 4 + 5 = 10 Step 3. Add these electrons to give every atom an octet